Hey there.
I'm assuming you know a little bit about what atoms, molecules and ions are, and you might know a bit of stoichiometry and the types of chemical reactions that happen in our crazy big/little world, and you're probably reading this because either you (a) are taking a course covering this topic, (b) you wish you took a course covering this topic or (c) you're simply interested!
I'm glad you're here.
Right now I'm taking a course at Wellesley College called CHEM 120: Intensive Introductory Chemistry with Laboratory, and I completed the Higher Level International Baccalaureate Chemistry course during my time in high school, so I believe I have sufficient knowledge to deliver a little crash course of my own.
Without further ado, read on as we delve into the topic of nuclear chemistry!
____________________________________________________________
Intro to the Atom
Matter is made up of atoms, and atoms are made up of nuclei and electrons. Different atoms have different compositions of subatomic particles in their nuclei.
The number of protons makes an element specific and identifiable. Protons are positively charged and the number of them present give the atomic number. Within a sample of atoms of an element, there can also be a different amount of neutrons, which do not affect the atomic charge or atomic number, but only atomic mass, and these variations of mass number are known as isotopes.
Collectively, protons and neutrons are called nucleons. The mass of these subatomic particles is measured in amu (atomic mass units).
How much exactly is an amu? It's defined as 1/12 of the mass of the carbon-12 atom, which turns out to be about 1.6605387 x 10^-27 kg.
Importance and Applications
Nuclear physics/nuclear chemistry is important because of the promise of nuclear fusion, which will provide clean, limitless energy for energy consumers in the world, but also because of the potential devastation that causes nervous tension and drama between enemy countries.
Scientists also are seeking a method to safely dispose nuclear waste to keep people safe from radioactive emissions.
PET (positron emission tomography) scans and MRI (magnetic resonance imaging) are in use by doctors to observe soft body parts and identify disease.
Geochemists and archaeologists measure the ages of rocks and archaeological artifacts by observing the decay of radioactive nuclides.
Mass Defect
I hope I have your attention now!
You would assume that the mass of a nucleus is just the sum of the masses of the neutrons and protons that it is made up of, but it's NOT.
I could give you a mathematical proof that this is so*, but I want this lesson to be fun and quick, so the key takeaway I want you to understand here is that the nucleus of any atom weighs less than it should.
Why is this so? Albert Einstein's famous equation, E=mc^2, equated mass and energy. The difference in mass is what allows the the neutrons and protons to "stick" together, and we call this the binding energy.
The binding energy of a nucleus is the negative of the energy change that would occur if that nucleus were formed from its component protons and neutrons
*Theoretically, we can calculate the mass that a carbon-12 nucleus should weigh and compare that to its actual mass, then multiply that by the speed of light, squared. This would give the binding energy in the units of Joules (J), which we can convert to electron volts (eV).
Structure and Stability in the Nucleus
We all learned in elementary school that opposites attract, and same charges repel.
This is the same in subatomic particles. Protons are repulsed by each other, so why don't the nuclei simply fly apart every time we put them together?
The repulsion is counterbalanced by the attractive strong nuclear force that acts between neutrons and nucleons (other neutrons and protons).
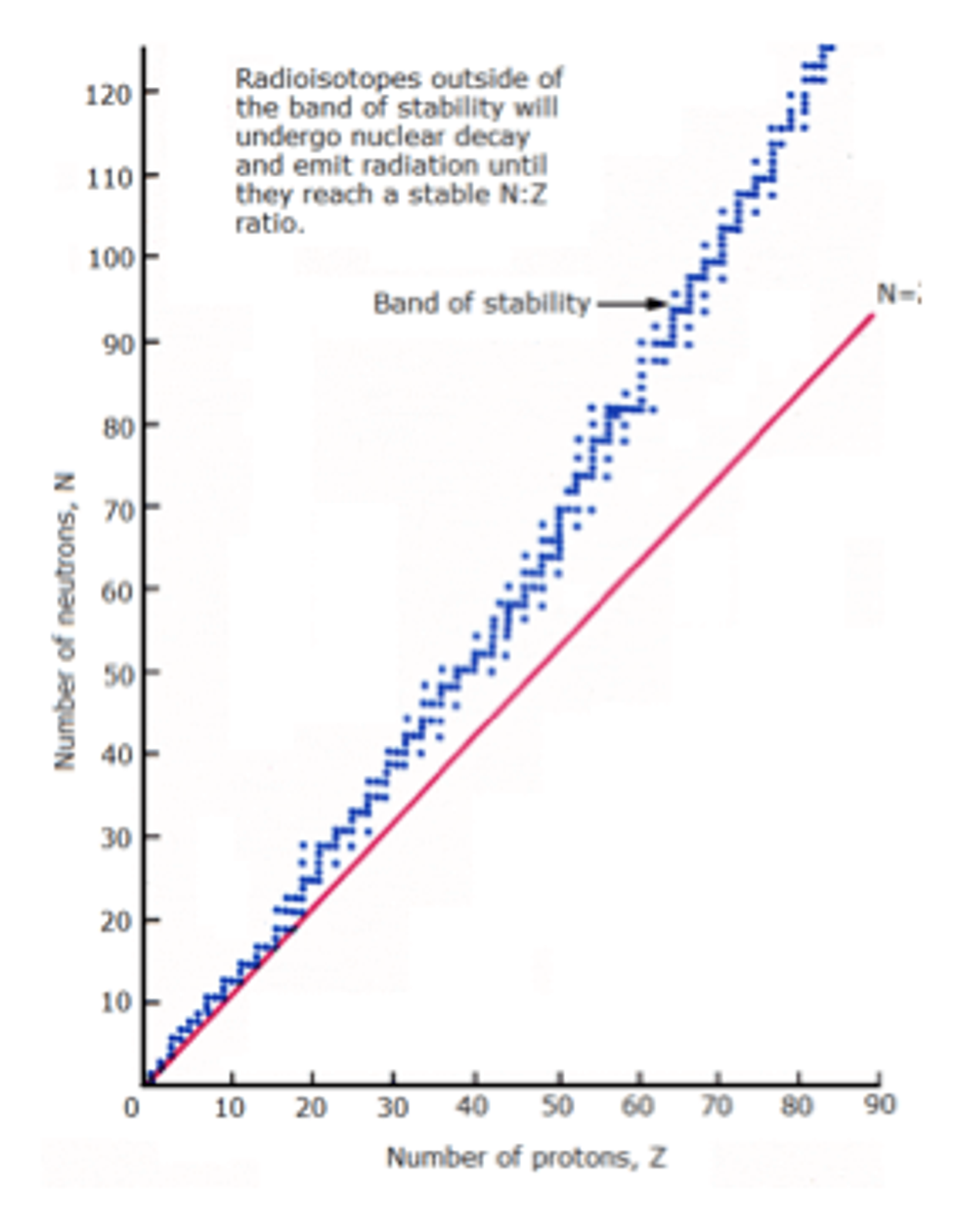
It's easy to imagine that the more nucleons that are in a nucleus, the more unstable it will be.
On this graph, the blue band represents the stable nuclides, shown relative to a 1:1 neutron-proton ratio. As the elements get heavier, the number of neutrons grows compared to the number of protons.
This is because that more neutrons are needed to counterbalance the forces between the number of protons, however, only a limited number of neutrons can provide this stabilizing influence, so we end up with this band.
Radioactive Decay
The purpose of radioactive decay is to allow a nucleus to become more stable.
Nuclides on the band do not need to undergo radioactive decay.
Nuclides on the right of the band have too many protons to be stable, so they will decrease the number of protons by spontaneous positron emission, or alternatively, electron capture.
Nuclides on the left of the band have too many neutrons to be stable, so they will undergo spontaneous beta decay.
Well, I suppose now we'll just have to dive straight in and learn about the following radioactive decay processes. (It's not as bad as it sounds.)
- Alpha Decay
- Beta Decay
- Gamma Rays
- Positron Emission (Beta Plus Decay)
- Electron Capture
Alpha particles are essentially helium nuclei. They have two protons and two neutrons and are emitted by nuclei which have too many protons and neutrons. Makes sense, right?
Beta particles are essentially electrons, only they are emitted from the nucleus. Everything else is the same. They have a -1 charge, and are emitted by nuclei with too few protons. Think about it, if you eject a -1 charge, you become more positive, see? Also, this does change atomic number but not the mass number, what really happens is that a neutron ejects an electron and becomes a proton.
Gamma rays are what turned Bruce Banner into the Hulk. Yes, they are electromagnetic radiation of high energy and short wavelength, emitted when the product radioisotope is above the nuclear ground state. It doesn't change atomic number or mass number.
Positrons are the antiparticles of electrons. Everything's the same, except the charge is flipped, so it's +1. They are emitted by nuclei below the band of stability, so that it increases the neutron/proton ratio and makes it more stable (that's the goal, remember!).
Electron capture is when a nucleus "eats" an electron, and converts a proton into a neutron. This also increases the neutron/proton ratio. Nope, it doesn't change mass number, but the atomic number decreases by one. It's the same result as positron emission!
For those of you who are attempting problems: Remember, that before and after these reactions, both mass number and atomic number must be conserved!
Sometimes, we will have a sequence of radioactive decays until we reach a stable nuclide.
Measuring Radioactivity
What happened to all that talk about radioactivity? We measure that stuff with Geiger-Muller counters, which you may or may not want to read about here or here (more advanced explanation). We can also use scintillation counters to make measurements.
Kinetics
Oo, what is this fancy word? Essentially we have something called the rate law, the rate equation, or the differentiated rate law. Whatever you want to call it.
Rate = -dN/dt = kN
What this is really saying is that the rate is decay is proportional to the number of nuclides in a sample. k is the decay constant, and is not influenced by temperature or pressure.
Activity, or A, can also be given similarly:
A = kN
If we integrate the differentiated rate law, we get the same equation written in another form.
ln N = -kt + ln N0
N / N0 = e ^ (-kt)
Activity is proportional to the number of nuclei (N) as well, so
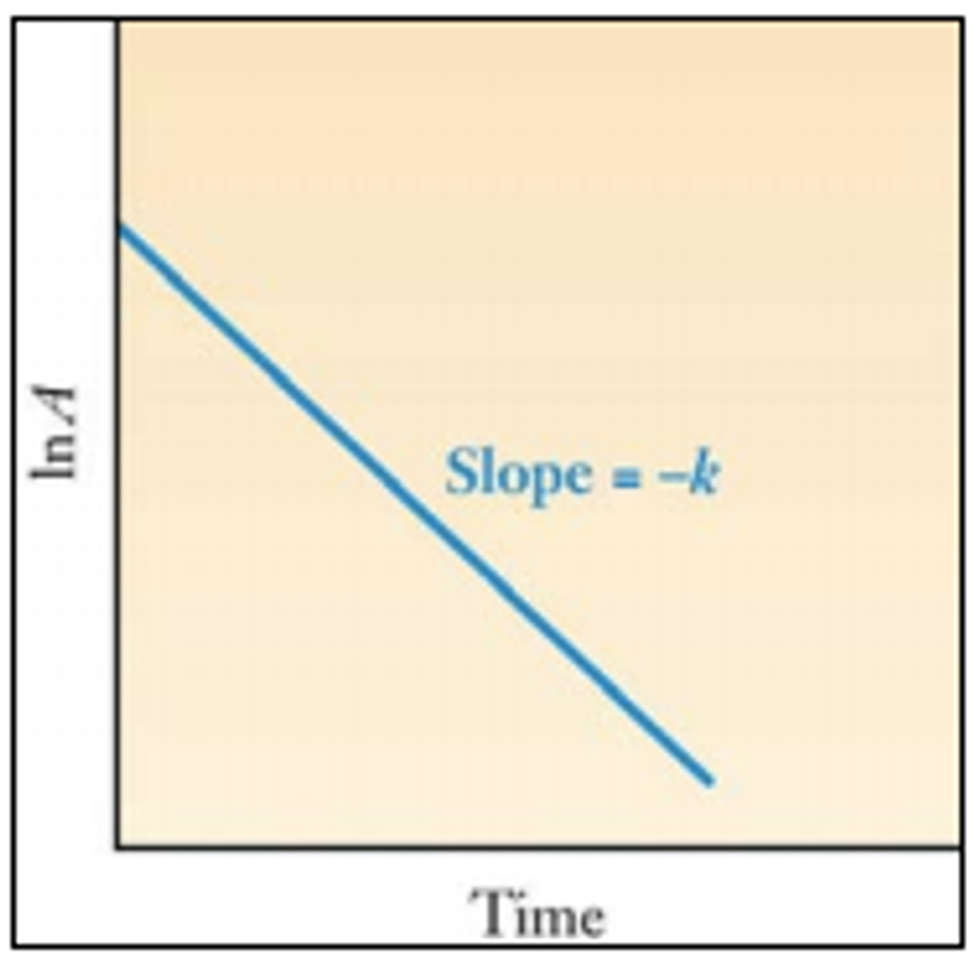
ln A = -kt + ln A0
Graphically, we can see these relationships when we plot ln A against time. The slope is the negative of the decay constant.
Now, let's talk about half-life. It's the time in which the number of nuclides decreases to half its original value.
...and that's how we relate half-life to the decay constant.*
The larger the decay constant, the shorter the half life, and vice versa.
*Mathematically, the number of nuclides at the time of one half life is N = N0 / 2. We substitute these values into the integrated rate law to get this equation.
Binding Energy per Nucleon
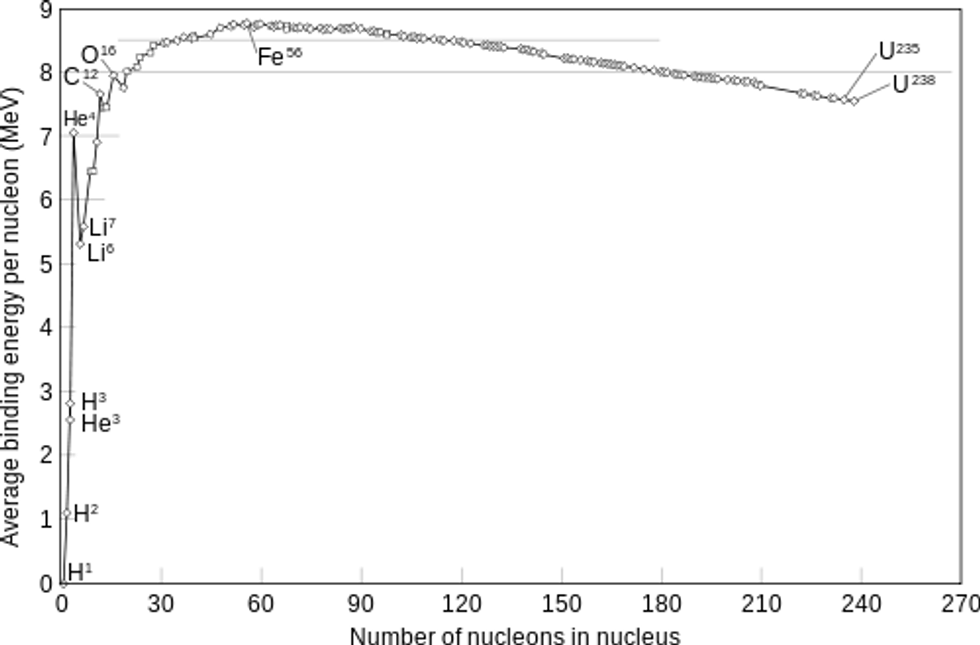
Remember when we talked about the binding energy of a nucleus? It turns out, when we look at the binding energy per nucleon (divide binding energy by the number of nucleons), it's actually quite an interesting pattern.
If you observe this graph, you will notice that Iron (Fe) has the highest binding energy per nucleon, which means that it is the most stable of all the nuclei. All nuclei want to be like Iron!
Previously we discussed one of the four types of nuclear reactions - nuclear decay. The other three are:
- Nuclear transformation by bombardment
- Nuclear fission
- Nuclear fusion and nucleosynthesis
This is the last stretch of our lesson so please stay with me!!
When a nucleus is bombarded with accelerated alpha particles, neutrons, or other nuclei, it can be transformed into another element! This can also be called nuclear transmutation.
Nuclear fission is the splitting of a nucleus into two approximately equal parts. During this, the number of neutrons can grow exponentially when neutrons released after each fission in turn set off another fission, and this chain reaction was utilized in the first atom bomb. Now, we use moderators to reduce the speed of neutrons and control these reactions.
Possible methods of disposing nuclear waste are: burial in deep mines (i.e. vitrification) or at sea, reprocessing or transmuting spent nuclear fuel, or launching into outer space.
Nuclear fusion is the combination of two light nuclides which form a heavier nuclide with the release of energy. This is where all the elements that exist naturally came from, except for H. The process happens in the stars (i.e. nucleosynthesis), and even heavier nuclei are produced in supernova explosions.
Our goal is to be able to do this, so we can have access to unlimited amounts of energy, but the conditions require a really high temperature so that the state of matter is plasma! It's borderline science fiction, but one day it could become reality.
And this is the end of this unit! I will be posting more in the weeks to come. If you enjoyed my crash course please subscribe to my blog. If there are any questions that I haven't answered please put them in the comments and I will definitely address them.
Thank you for reading! Don't forget that learning is a joy.