One day, I was eating lunch with my girlfriend. We were quietly eating until I broke the silence with "I can't remember what gas they use to preserve the Declaration of Independence." This comment had nothing to do with any prior conversation; it was just a product of my curiosity. Neither of us could remember exactly what was used to preserve the historical document, but we knew that helium was used at some point.
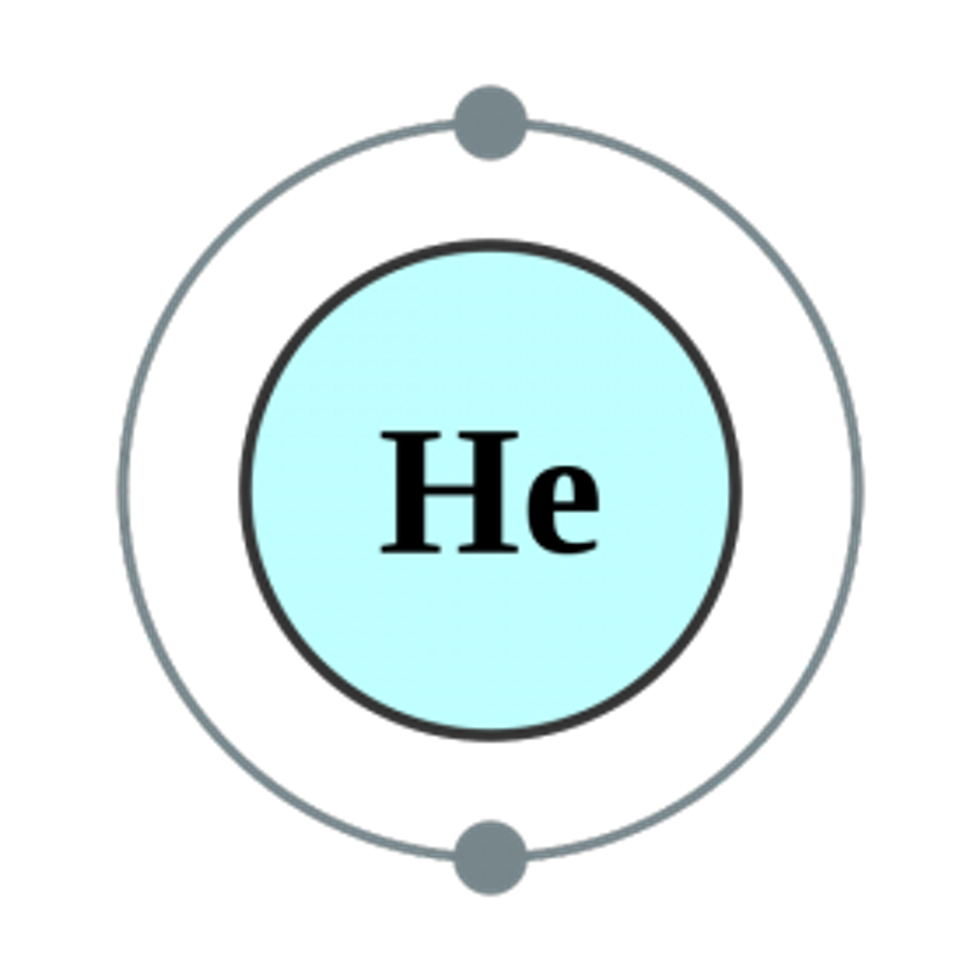
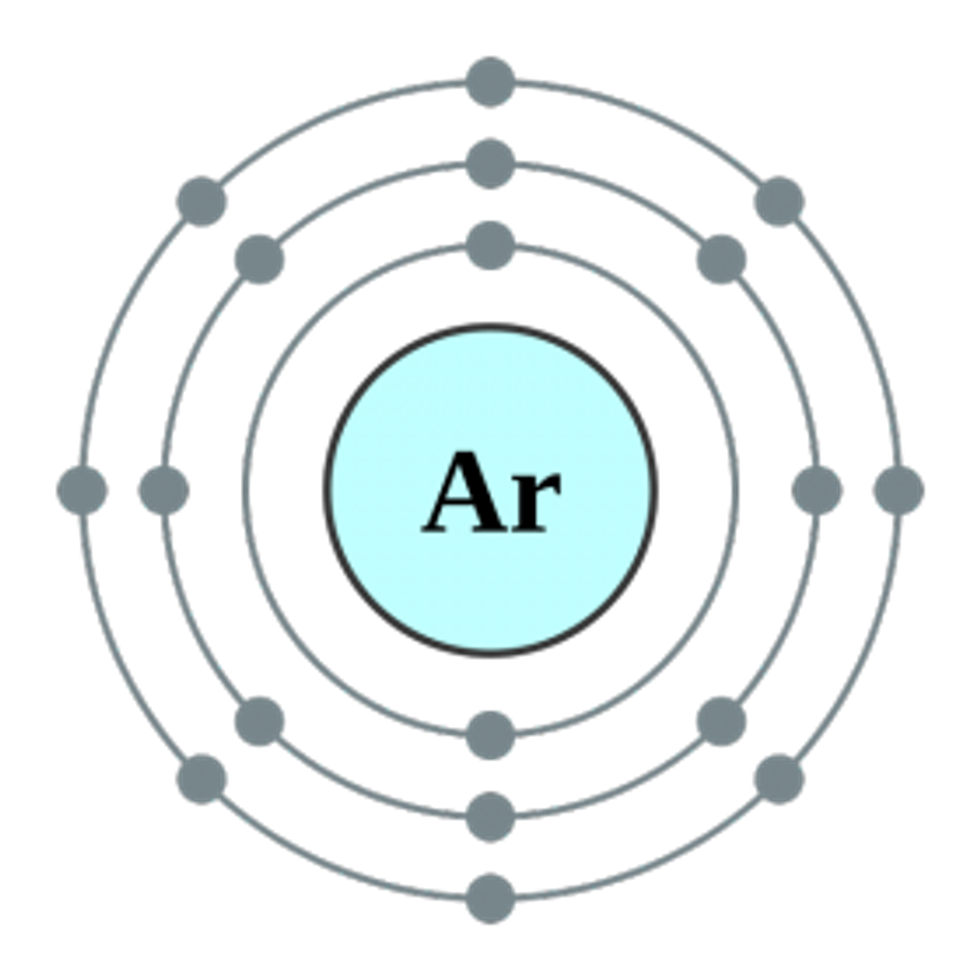
The two gases that popped into my head were argon and hydrogen. Hydrogen is very highly reactive, so I knew that it most likely was not the gas preserving one of the most iconic documents in American history. Argon is a noble gas, just like helium. So which is it? Did they stop using helium or did they decide it was good enough to protect the Declaration?
I did some research after a few days because not knowing the answer started to bug me, and this is what I found.
According to the U.S. National Archives and Records Administration, the Declaration is encased with argon under a controlled humidity. There's the first half of the answer. But what caused the change? According to the Prologue Magazine of the National Archives, the document was encased with helium until 2003. The humidity of the helium upon encasement was 30 percent. Upon the opening of the encasement in 2003, the humidity was evidently greater than 40 percent. Was the humidity change just a problem with the casing or a problem with the noble gas?
You would probably think that helium and argon are almost the same thing, since they are both noble gases, but the size difference alone is enough to consider some of their other properties.
WebElements does a good job of putting all the properties of these noble gases all in one place. Helium is very nonreactive, but can dissolve slightly in water to a small extent. Helium is very small and can escape most containers, even if they are sealed. Argon is also very nonreactive, and also dissolves in water - but it dissolves to a much lesser extent than helium does.
The bigger the noble gas, the better, right? Actually, there's a problem with some of the larger noble gases, too. Even though they aren't very reactive, krypton and xenon have the capacity to react with fluorine to create compounds. The larger the atom, the less energy is required to strip the outermost electrons from it.
With that in mind, argon is the best candidate to preserve old and aging documents. Right in the middle of too-small-and-could-possibly-escape-the-encasement and too-large-and-is-potentially-reactive-under-the-right-circumstances.
There are copies of the Declaration of Independence, though. The written document has been archived electronically and many copies have been distributed to various places throughout the country. Is it absolutely necessary to put so much effort into preserving the original document? Or is all of this talk of noble gases inside the document's encasement just full of hot air?